Nanoplastics pollution found to make foodborne pathogens more dangerous
Researchers have found that nanoplastics make foodborne bacteria like E. coli O157:H7 stronger, increasing their dangers to people.
According to the report, nanoplastics are everywhere. The microscopic fragments can accumulate on bacteria and be taken up by plant roots and are in water and food. The particles are from plastic pollution such as foam containers used for takeout food.
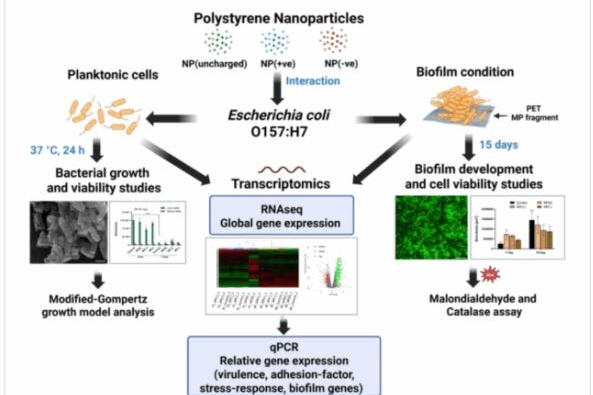
New research from scientists at the University of Illinois Urbana-Champaign shows certain nanoplastics make foodborne pathogens more virulent, which increases the danger of more severe illnesses in people. The study, “Nanoplastics-mediated physiologic and genomic responses in pathogenic Escherichia coli O157:H7,” is published in the Journal of Nanobiotechnology.
“Other studies have evaluated the interaction of nanoplastics and bacteria, but so far, ours is the first to look at the impacts of microplastics and nanoplastics on human pathogenic bacteria. We focused on one of the key pathogens implicated in outbreaks of foodborne illness — E. coli O157:H7,” said senior study author Pratik Banerjee, associate professor in the department of food science and human nutrition and an Illinois Extension Specialist; both units are part of the College of Agricultural, Consumer and Environmental Sciences at the University of Illinois.
Banerjee’s team found that nanoplastics with positively charged surfaces were more likely to cause physiological stress in E. coli O157:H7.
“Just as a stressed dog is more likely to bite, the stressed bacteria became more virulent, pumping out more Shiga-like toxin, the chemical that causes illness in humans,” according to the researcher.
The researchers expected positively charged nanoplastics to impact E. coli because the bacteria’s surface carries a negative charge. To test their opposites-attract hypothesis, they created nanoplastics from polystyrene, commonly used for food containers, and applied positive, neutral or negative charges before introducing the particles to E. coli either free-floating in solution or in biofilms.
“We started with the surface charge. Plastics have an enormous ability to adsorb chemicals. Each chemical has a different effect on surface charge, based on how much chemical is adsorbed and on what kind of plastic,” Banerjee said. “We didn’t look at the effects of the chemicals themselves in this paper — that’s our next study — but this is the first step in understanding how the surface charge of plastics impacts pathogenic E. coli response.”
The scientists found that bacteria exposed to positively charged nanoplastics were changed in several ways. They formed biofilms more slowly but rebounded and became more hearty.
Biofilms give bacterial cells a measure of protection because of an extracellular coating they develop. They are notoriously difficult to eradicate in food processing facilities including salad production plants and plants to produce processed foods.
To test whether biofilms protected against nanoplastic-induced stress, the research team dunked comparatively large microplastic particles into the bacterial soup they created and gave E. coli a week or two to colonize. Then, they introduced the same charged nanoplastics. The positively charged particles still caused stress — and enhanced Shiga-like toxin production — in biofilm-bound E. coli.
“Biofilms are a very robust bacterial structure and are hard to eradicate,” Banerjee said. “One of our goals was to see what happens when this human pathogen, which is commonly transmitted via food, encounters these nanoplastics from the vantage point of a biofilm.”
Banerjee’s research team has studies underway to look at resistance gene transfer and changes in virulence and transmission patterns of major foodborne pathogens in food products and other environments such as soil.
Banerjee’s research was supported in part by a U.S. Department of Agriculture National Institute of Food and Agriculture grant.
The entire research report can be found here.